Simple
Provides an intuitive,
easy-to-operate user experience
Affordable
Flexible and customer-
friendly placement program
Rapid
15 minutes to result*
Accurate
Lab-quality PCR results
at the point of care
Multiplex
Multiplex-capable in a
single test cartridge
Room temperature storage
Including test cartridges,
collection kits, and controls**
Multiple sample types
Designed to support a wide
variety of sample types
Intuitive patient report
Wi-Fi and cloud connectivity enabled
Watch the demo
How to run a test
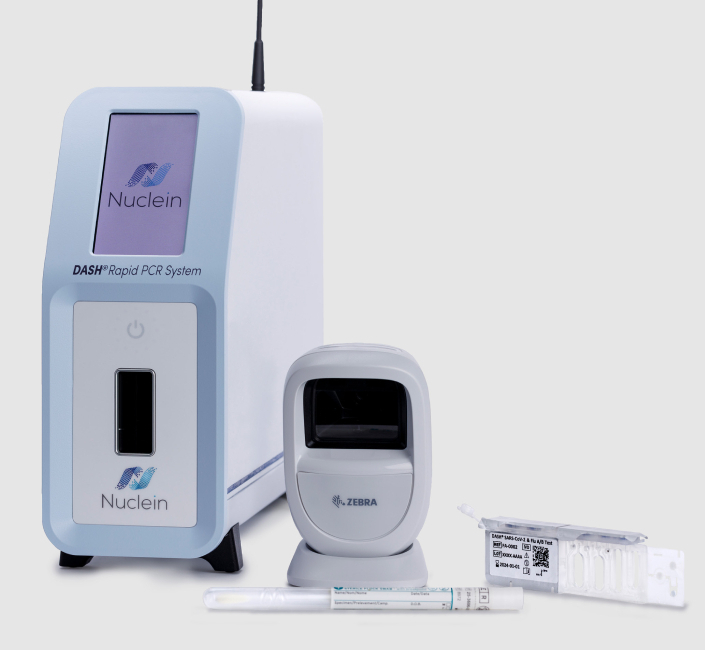
DASH Rapid PCR System

The DASH Instrument is a sample-to-answer system that automates the process of sample preparation, sequence-specific capture and PCR amplification, detects the presence of target pathogens, and reports the result. The instrument weighs less than 10 pounds and has a very small footprint that allows it to fit on a desktop in a wide variety of care settings, including hospitals, urgent care, outpatient clinics, emergency rooms, physician’s offices, and decentralized labs. The DASH Instrument requires no regular maintenance.
The DASH test cartridge contains all reagents necessary to perform a test and is built to be multiplex capable. DASH test cartridges, sample collection swabs, and controls are stored at room temperature.**
The DASH Rapid PCR System delivers lab-quality results at the point of care, is simple to use, and can be operated with minimal training.
DASH Rapid PCR System allows for
testing and treating in a single patient visit
Test workflow
**Room temperature (15-30°C) storage for test cartridges and controls



